Thank you for your quick response. Curtis your help and past year problem will save us fawn loss. Ross thank you for sharing fuso guard with us. Dawn is on her way to your ranch to pick up any extra. We are giving penicillin to all bottle fawns. Our vet did culture test and should have results Monday. Great to have such wonderful support. Thank you Isaac his Dad (Scott) and the Applecreek team!!!
Introduction
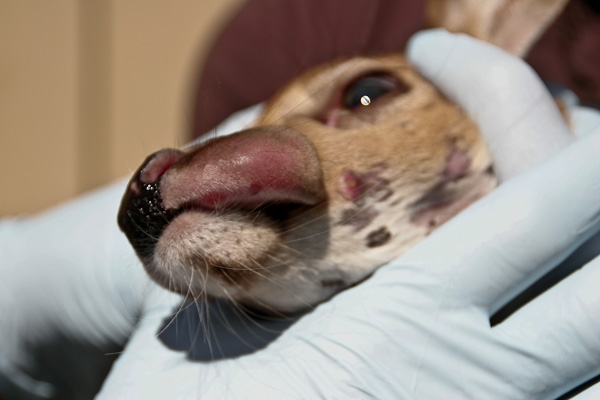
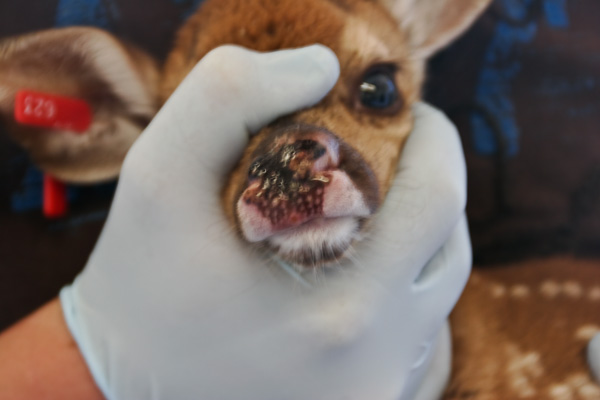
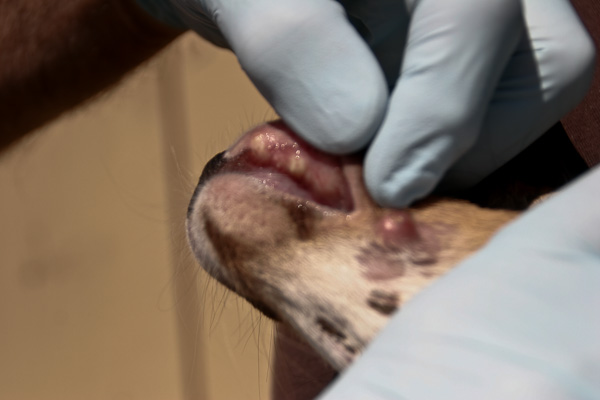
Necrobacillosis is also known as necrotic stomatitis, hepatic necrobacillosis, foot rot, calf diphtheria and lumpy jaw. It produces a variety of diseases, with abscessation occurring in almost any body organ or joint cavity. In white-tailed deer it is usually seen as individual cases of lumpy jaw or as a fatal infection of multiple fawns.
Cause
Necrobacillosis is caused by Fusobacterium necrophorum, an anaerobic, gram negative, often highly filamentous rod-like bacteria. Being "anaerobic" means that it likes to grow in places where there is very little air or oxygen. This organism is found in the intestines of many species as part of the normal flora. It survives well in wet soil that has a high manure content. Other bacteria are often associated with the disease, Arcanobacterium pyogenes being the most common.
Transmission
Animals develop necrobacillosis when a number of factors are present. Stress such as heat, cold, overcrowding or poor nutrition predispose to infection. Fusobacterium cannot penetrate intact skin. The organism gains entry into the body through cuts or abrasions to the skin or mucous membranes. In the mouth, grass awns, coarse feed, metal objects, and unevenly worn or newly erupting teeth can penetrate or damage the oral lining and create points of entry. Contamination of the abrasions with soil containing F. necrophorum results in an infection. Changes in the rumen environment such as grain overload or sudden changes in feed can damage the stomach lining. This provides a portal of entry for Fusobacterium, where they may then pass to the liver, causing hepatic necrobacillosis, or to other major organs.
In some situations the disease appears to be contagious and there is, no doubt, direct contamination of shared feed bunks and drinking water by infected animals with open lesions. The infectivity or invasiveness of F. necrophorum is enhanced by the presence of other bacteria such as E. coli and A. pyogenes and the presence of these bacteria permit infection by relatively few F. necrophorum organisms (9).
Other species affected
Cases of necrobacillosis have been reported in many species of wild and domestic hoofed animal. In farmed white-tailed and fallow deer, necrobacillosis is recognized as a serious problem of young or newly weaned fawns. There have been large die offs of wild mule deer and white-tailed deer, reindeer and elk (7). Heavy losses of kangaroos and wallabies have occurred in zoos and the wild (4). Foot rot can be a significant problem in cattle, sheep and bison and also wild hoofed stock (6).
Clinical signs
The location in the body of lesions determine what signs are seen. Affected animals are usually depressed and have a fever. Often the hair coat is rough and the animal is thin and doing poorly. Lesions involving the mouth are often deep, invading the surrounding soft tissue and bone creating the classic swollen jaw or face. Animals may go off feed and begin to lose condition as the problem develops. In fawns the lack of nutritional reserve and toxic by-products of the infection lead to a more rapid death.
Lameness is seen when the feet and associated joints are involved. Swelling between the toes is the first sign seen, followed by localized tissue death, spreading to the joints and bones in more advanced cases (5,7,10).
In the throat, necrotic laryngitis will show itself as loud wheezing. Some dead tissue and bacteria may be sucked into the lungs causing abscess formation and pneumonia.
Cases of necrotic rumenitis or stomach abscesses frequently have few signs except weight loss in chronic forms or apparent sudden death.
Post mortem findings
The most common lesions are necrotic ulcers or abscesses of varying depth on oral, pharyngeal (throat), or laryngeal mucous membranes. In severe cases, lesions extend into the nasal cavity, upper and lower jaw bones, larynx, trachea and lungs. Spread of F. necrophorum through the bloodstream may cause abscesses in internal organs. The abscesses contain foul smelling, thick purulent material having a greenish tinge. Rumen lesions are characterized by well defined, yellow, raised foci of necrosis. These extend deep into the rumen wall and can perforate, leading to peritonitis, or infection of the abdominal cavity (10).
Diagnosis
Clinical signs and history are usually sufficient to establish a diagnosis. The organism is difficult to grow, making culture and isolation difficult without special culture swabs and growth medium. Identification of the organism in lesions can be made with a flourescent antibody test (7). There is also a blood test (ELISA) for the detection of serum antibodies to F. necrophorum in sheep and cattle (2). However, these tests may not be presently available to all pathology laboratories.
Treatment
Early detection of necrobacillosis is important if the disease is to be treated. The long term results of treatment are not encouraging. Affected animals should be isolated from healthy animals. Feeding and drinking areas and working facilities should be cleaned and disinfected to prevent spread of the organism. Antibiotics are indicated for therapy. Although, like most drugs, they are not currently licensed for use in deer. Procaine penicillin is likely most effective with tetracyclines and sulfonamides being used as well. Where possible abscesses should be drained and flushed to remove debris and toxins. Debris and pus from the lesion should not be allowed to contaminate the treatment area. Collect and incinerate the material in a plastic bag. Amputation of a toe may be required in severe cases of foot rot where there is bone and joint involvement.
Prevention
As with most diseases, prevention is much more rewarding than treatment. Good management practices form a large part of any control or prevention program.
* Stress from overcrowding, social or hierarchy instability, inappropriate mixing of sexes and ages, or poor animal handling technique should be minimized.
* Good general hygiene should be practised with attention paid to control of excessive fecal contamination of paddocks, holding and handling facilities, and feeding equipment. Hay offered on the ground will become contaminated with urine and feces.
* Do not use preventative antibiotics without a diagnosis or good evidence of infection. In the long term, more problems will be created than will be prevented.
* Food structure (coarseness) is probably not as important as the nutritional quality of food. Poor quality food decreases resistance to disease in general.. There is some evidence that mucosal skin integrity depends on adequate vitamin A and C levels (4). Deficiency of these vitamins may predispose animals to necrobacillosis infection by making invasion easier.
* The type of soil underfoot may play a role in the persistence and transmission of F. necrophorum. Clay and other water retaining types of soil are thought to support bacterial life better than well drained sandy soils (4). Whatever the soil type, avoid poorly drained paddocks and moist areas to raise deer.
Vaccination
Vaccination will never replace good management practices. However when faced with persistent disease vaccination may be an option. There is no commercial vaccine licensed for prevention of necrobacillosis in deer but variable results have been observed with Fusogard™ (Novartis). Fusogard™ is a pure F. necrophorum bacterin designed to prevent footrot and liver abscesses in cattle. Some deer producers claim to have achieved good protective results and others have been disappointed. Its usefulness in deer species has never been scientifically established.
Because necrobacillosis is most often a mixed bacterial infection (F. necrophorum plus others), it may be that every farm outbreak involves a unique combination of bacteria, similar but different from each other. The use of "autogenous" vaccines may therefore hold more promise. Autogenous bacterins are made up separately for each infected farm from bacteria derived from infections on that particular premises. (Auto genous = self generated)
Animals are inoculated with a product made up from bacteria obtained from lesions on that farm, creating a more specific and effective immunity to the necrobacillosis organisms. Autogenous vaccines have been used to treat necrobacillosis in sick animals as well as prevent disease in healthy ones. Both uses have not been adequately tested in white-tailed deer and further investigation is needed to determine their usefulness.